Cardiac Amyloidosis
Amyloidosis is a rare disease characterized by the buildup of amyloid deposits in parts of the body. Cardiac amyloidosis occurs when the amyloid deposits build up in the heart muscle, impairing the heart’s ability to pump blood effectively. The most common types of cardiac
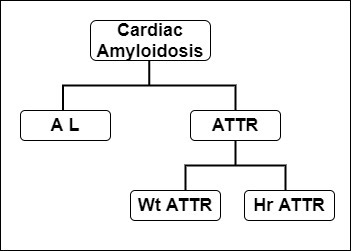
amyloidosis are AL amyloidosis and ATTR-CM. ATTR-CM is further subdivided into wild-type ATTR-CM and hereditary ATTR-CM.
Cardiac amyloidosis is of particular interest and concern because heart complications are the most common cause of death in patients with amyloidosis. Without treatment, median survival for AL amyloidosis is less than six months and 3 to 5 years for transthyretin amyloidosis.
(Dorbala et al., 2020).
Patients with cardiac amyloidosis often experience misdiagnosis or delays in diagnosis. A recent literature review found that 34-57% of patients with ATT-CM receive misdiagnoses such as hypertensive heart disease, hypertrophic cardiomyopathy, and ischemic heart disease.
(Rozenbaum et al., 2021).
The same review found delays in diagnosis ranging from 2.6 to 6.1 years (Rozenbaum et al., 2021).
Delayed cardiac amyloidosis diagnosis is associated with increased cardiac symptoms and poor quality of life (Bishop et al., 2018).
More opportunities for non-invasive screening, such as genetic testing and bone radiotracer scintigraphy, have become available in recent years. New pharmacological treatments have also received FDA approval.
Cardiac amyloidosis can cause a range of symptoms, including:
Fatigue
Shortness of breath during exercise or other physical activities
Shortness of breath while lying down
Feeling faint or light-headed
Swelling in the legs
Abdominal distension or "swelling."
There are standard tests in the diagnostic journey of Cardiac amyloidosis
Electrocardiogram:
Nuclear SPECT:
Endomyocardial biopsy:
Genotyping:
While diagnosing cardiac amyloidosis may sound worrisome, various effective treatments are available. Early diagnosis is the key to determining prognosis. A high degree of suspicion based on the clinical history and laboratory investigations can lead to earlier diagnosis and quicker initiation of therapy. The prognosis of patients with AL amyloidosis has dramatically improved over time, with a four-year overall survival of approximately 90% for successfully treated patients with stem cell transplantation in the contemporary era.
Our goal is to:
● increase awareness and understanding of disease epidemiology.
● increase awareness and understanding of diagnostic tools available for diagnosis,
including genetics, biopsy, and bone radiotracer scintigraphy.
● increase awareness and understanding of pharmacologic and nonpharmacologic
disease management.
● increase awareness and understanding of the differential diagnosis of
cardiomyopathies.
Overall, cardiac amyloidosis is a rare disorder. The prevalence varies with the etiological type. About 10% of multiple myeloma cases may have AL amyloidosis, and 50 to 70% may have cardiac involvement. The annual incidence of AL amyloidosis is 1 per 100,000
In AL amyloidosis, amyloid protein is derived from immunoglobulin light chains, and most often involves the kidneys and the heart. ATTR amyloidosis is categorized as mutant or wild-type depending on the genetic sequence of the transthyretin (TTR) protein produced by the liver.
Wild-type ATTR amyloidosis mainly involves the heart, although the reported occurrence of bilateral carpal tunnel syndrome, spinal stenosis and biceps tendon rupture in these patients speaks to more generalized protein deposition.
Mutant TTR is marked by cardiac and/or peripheral nervous system involvement.
Cardiac involvement is associated with symptoms of heart failure and dictates the clinical course of the disease.
Siddiqi OK, Ruberg FL. Cardiac amyloidosis: An update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018 Jan;28(1):10-21.
Shams P, Ahmed I. Cardiac Amyloidosis. [Updated 2022 Jul 1].
Muchtar E, Jevremovic D, Dispenzieri A, Dingli D, Buadi FK, Lacy MQ, et al. The prognostic value of multiparametric flow cytometry in AL amyloidosis at diagnosis and at the end of first-line treatment. Blood. 2017;129(1):82–7.
