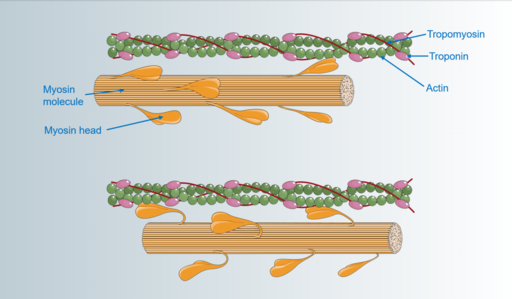
Many people first discover they have HCM because blood flow out of their heart is obstructed. Some of these patients eventually need to have their septums reduced physically by surgery ( septal myectomy ) or alcohol septal ablation. Can a drug replace these invasive treatments?
On April 2, 2022 Dr. Milind Y. Desai , a leading HCM researcher and practitioner, will present initial results on mava camten ‘s use in severely obstructed HCM patients. Dr. Desai is Director of the HCM Center at the Cleveland Clinic. Scheduled for the American College of Cardiology meeting in Washington. His talk on “Mavacamten as an alternative to surgical septal myectomy or alcohol ablation in patients with severely symptomatic obstructive hypertrophic cardiomyopathy” will present results from the VALOR-HCM trial.
This will be the first set of results on mavacamten’s use in HCM patients who are obstructed. For further background on mavacamten, see this interview with Dr. Martin S. Maron.
The post Mavacamten instead of septal reduction? appeared first on Hypertrophic Cardiomyopathy Association.
HCMA Blog


 Translate
Translate
