HCM Medication
About Mavacamten
(trade name Camzyos)
Mavacamten is a first-in-class, oral, allosteric modulator of cardiac myosin for the treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy was developed by Myokardia and purchased by Bristol Myers Squibb.
Mavacamten aims to tackle HCM at its source rather than managing symptoms. Research is underway for other types of heart disease, including non-obstructive HCM and heart failure with preserved ejection fraction. Bristol Myers Squibb is also testing whether the drug can spare patients from surgical procedures.
Mavacamten may reduce the number of people who need to go on to have SRT, achieved using surgery or a catheter-based procedure, which is a standard therapy for severe HCM.
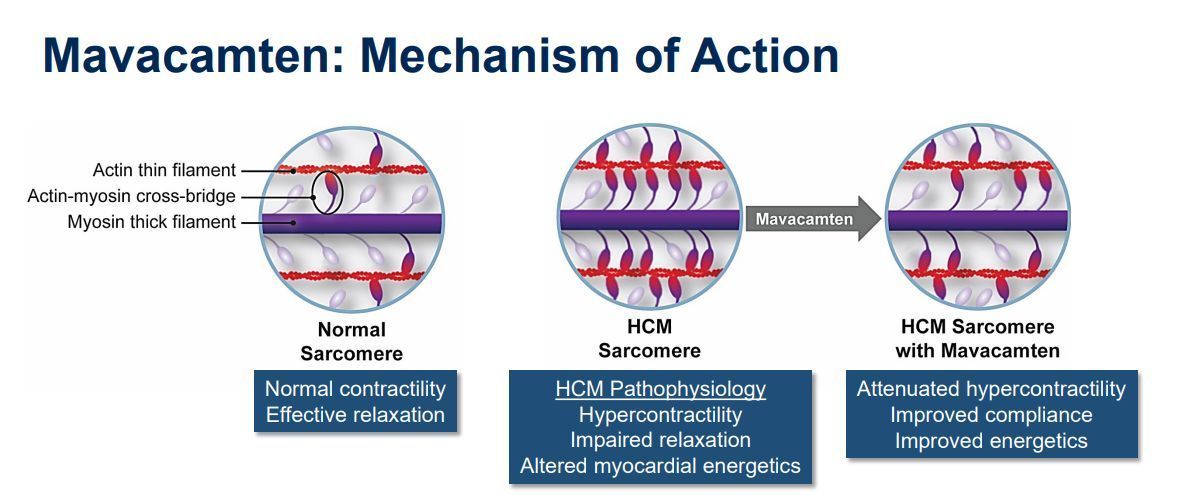
The mechanism of action for mavacamten, a cardiac myosin inhibitor to treat obstructive hypertrophic cardiomyopathy.
Source: https://www.dicardiology.com/content/mavacamten-effective-treating-obstructive-hypertrophic-cardiomyopathy
Bristol Myers Squibb Information
BMS provided links related to Camzyos:
Online Information click here: CAMZYOS PATIENT PORTAL
Camzyos - Telephone support line for HCMA Members: 888-694-2059
The information line can provide answers to questions about access and the specific programs available through the MyCamzyos program.
CAMZYOS Medication Guide
CAMZYOS U.S. Prescribing Information
CAMZYOS REMS Program
To determine eligibility and apply for financial assistance, visit
HealthWell’s Cardiomyopathy – Medicare Access Fund page.
Watch the special edition of our Bighearted Warrior Tour where we speak with members of the Camzyos™ team at Bristol Myers Squibb to better explain:
- Label Indication
- Patient Assistance Program
- Medicare Access
Subsidy Information
Medicare Part D
Gavin Clingham with Alliance for PAtient Access (AfPA) explains Medicare benefits.
My sincere thanks to all who have been a part of this important chapter of HCM history. I end with a word of balance and cautious optimism. We will learn together the effectiveness, sustainability, and role of myosin inhibitors for the HCM community. On your marks, get set, go – cautiously into the future together.
- Lisa Salberg
“Adding mavacamten to maximally tolerated medical therapy significantly reduced patients’ eligibility for and/or desire to proceed with septal reduction therapy,” says Dr. Desai, Director of the Hypertrophic Cardiomyopathy Center at Cleveland Clinic. “No approved medical therapies have been developed specifically for hypertrophic cardiomyopathy or evaluated in randomized controlled trials for this condition, so mavacamten shows promise to address an unmet need for a non-invasive treatment in this setting.”
Source: https://consultqd.clevelandclinic.org/mavacamten-significantly-reduces-patients-need-for-septal-reduction-therapy-over-16-weeks/
The listing of the countries where Camzyos is approved for symptomatic obstructive hypertrophic cardiomyopathy (HCM) in adult patients.
- Rader F, Choudhury L, Saberi S, et al. Updated cumulative results of treatment with mavacamten from the EXPLORER-LTE cohort of the MAVA-LTE study in patients with obstructive hypertrophic cardiomyopathy. Presented at: ACC 2022. April 3, 2022. Washington, DC.
- Hegde SM, Lester SJ, Solomon SD, Michels M, Elliott PM, Nagueh SF, Choudhury L, Zemanek D, Zwas DR, Jacoby D, Wang A, Ho CY, Li W, Sehnert AJ, Olivotto I, Abraham TP. Effect of Mavacamten on Echocardiographic Features in Symptomatic Patients With Obstructive Hypertrophic Cardiomyopathy. J Am Coll Cardiol. 2021 Dec 21;78(25):2518-2532. doi: 10.1016/j.jacc.2021.09.1381.
- Xie J, Wang Y, Xu Y, Fine JT, Lam J, Garrison LP. Assessing health-related quality-of-life in patients with symptomatic obstructive hypertrophic cardiomyopathy: EQ-5D-based utilities in the EXPLORER-HCM trial.
- J Med Econ. 2022 Jan-Dec;25(1):51-58. doi: 10.1080/13696998.2021.2011301.
Burstein Waldman C, Owens A. A plain-language summary of the EXPLORER-HCM study: mavacamten for obstructive hypertrophic cardiomyopathy. Future Cardiol. 2021 Oct;17(7):1269-1275. doi: 10.2217/fca-2021-0044. Epub 2021 May 21. - Spertus JA, Fine JT, Elliott P, Ho CY, Olivotto I, Saberi S, Li W, Dolan C, Reaney M, Sehnert AJ, Jacoby D. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021 Jun 26;397(10293):2467-2475. doi: 10.1016/S0140-6736(21)00763-7. Epub 2021 May 15.
- Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, Saberi S, Lakdawala NK, Wheeler MT, Owens A, Kubanek M, Wojakowski W, Jensen MK, Gimeno-Blanes J, Afshar K, Myers J, Hegde SM, Solomon SD, Sehnert AJ, Zhang D, Li W, Bhattacharya M, Edelberg JM, Waldman CB, Lester SJ, Wang A, Ho CY, Jacoby D; EXPLORER-HCM study investigators. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020 Sep 12;396(10253):759-769. doi: 10.1016/S0140-6736(20)31792-X. Epub 2020 Aug 29. Erratum in: Lancet. 2020 Sep 12;396(10253):758.Ho CY, Olivotto I, Jacoby D, Lester SJ, Roe M, Wang A, Waldman CB, Zhang D, Sehnert AJ, Heitner SB. Study Design and Rationale of EXPLORER-HCM: Evaluation of Mavacamten in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy. Circ Heart Fail. 2020 Jun;13(6):e006853. doi: 10.1161/CIRCHEARTFAILURE.120.006853. Epub 2020 Jun 5.


 Translate
Translate