SEQUOIA-HCM
A Clinical Trial Evaluating an Investigational Drug for Obstructive Hypertrophic Cardiomyopathy (oHCM)
BACKGROUND
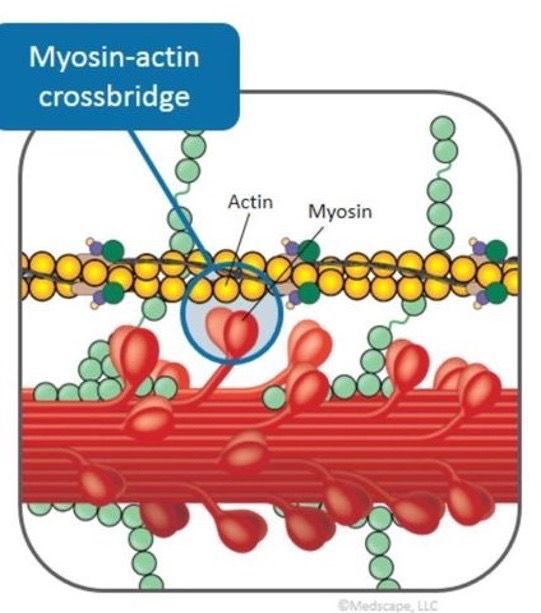
Hypertrophic cardiomyopathy (HCM) is a chronic heart disease in which the abnormally thickened (hypertrophied) heart muscle (myocardium) prevents the heart from pumping blood efficiently (cardiomyopathy).
Obstructive hypertrophic cardiomyopathy (oHCM) is a condition that may occur in people who have HCM. oHCM is a condition in which the abnormally thickened heart muscle partially blocks the blood flow out of the heart forcing the heart to work harder to pump blood to the body.
Patients diagnosed with oHCM may not exhibit symptoms in the early stages of the condition but may develop them over time, including signs and symptoms that can disrupt daily life, such as:
- Shortness of breath
- Chest heaviness or pain
- Dizziness and fainting
The severity of these symptoms varies widely between people with oHCM and can even vary day-to-day.
Currently, this form may only be submitted by people in the United States. If you live outside of the United States, please contact your physician for additional information and further guidance.
About SEQUOIA HCM: A PHASE 3 CLINICAL TRIAL
SEQUOIA-HCM is currently recruiting participants to evaluate the efficacy and safety of the investigational drug, aficamten, in patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM).
People with symptomatic oHCM may qualify. There is no cost to participate in the study. If you qualify, the investigational drug, study related tests, assessments, and visits will be provided at no cost. Trial participants may be reimbursed for study-related travel.
KEY PARTICIPATION CRITERIA:
- You have been diagnosed with oHCM
- You are between 18 to 85 years of age
- You still have symptoms related to your heart condition even though you are on medications
- You have not received prior treatment with this study drug
- You have not participated in another investigational device or drug trial
- You are not pregnant or breastfeeding
Additional participation criteria will apply. Further details regarding the participation criteria can be found at https://clinicaltrials.gov/ct2/show/NCT05186818.
CLINICAL TRIAL PARTICIPATION PERIOD: Now and ongoing.
SEQUOIA HCM: Commonly asked questions FAQ
Olivotto I. Int J Cardiol. 2018. 15;251:71-73
Green et al. Science. 2016; 351:617

 Translate
Translate